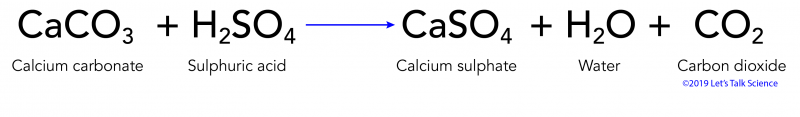
What is calcium carbonate + sulfuric acid as a word equation and a balanced symbol equation? - Quora

25 gram of calcium carbonate completely reacts with sulphuric acid to produce 11g of carbon dioxide. the - Brainly.in

Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution

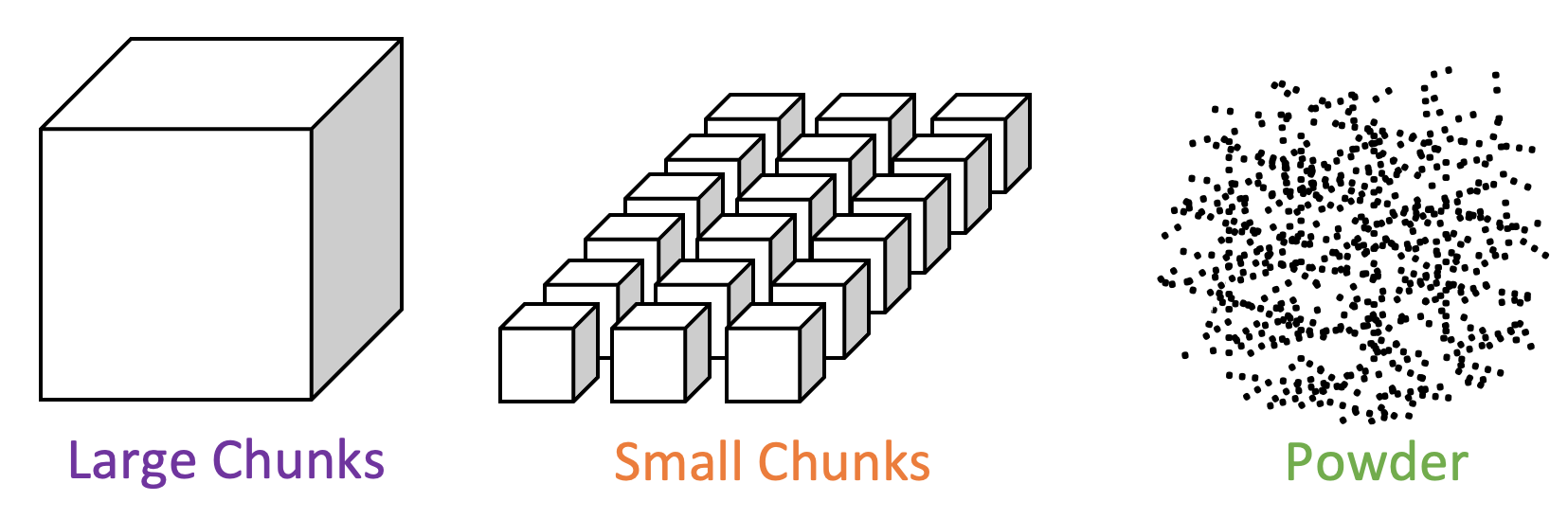
reactivity - How could mass increase when sulfuric acid is added to calcium carbonate? - Chemistry Stack Exchange

28.5 g of calcium carbonate (CaCOn reacts with an excess of sulfuric acid (H SOg) to farm calcium sulfate - brainly.com
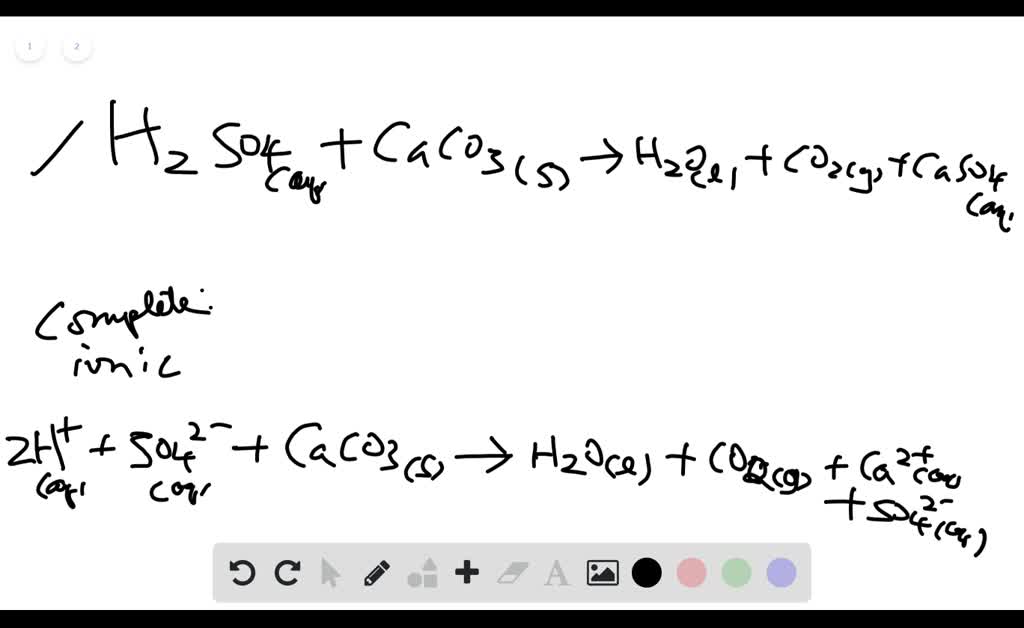
SOLVED: Write complete ionic and net ionic equations for the reaction between sulfuric acid (H2 SO4) and calcium carbonate (Ca CO3) . H2 SO4(aq)+ CaCO3(s) →H2 O(l)+CO2(g)+CaSO4(aq) | Numerade

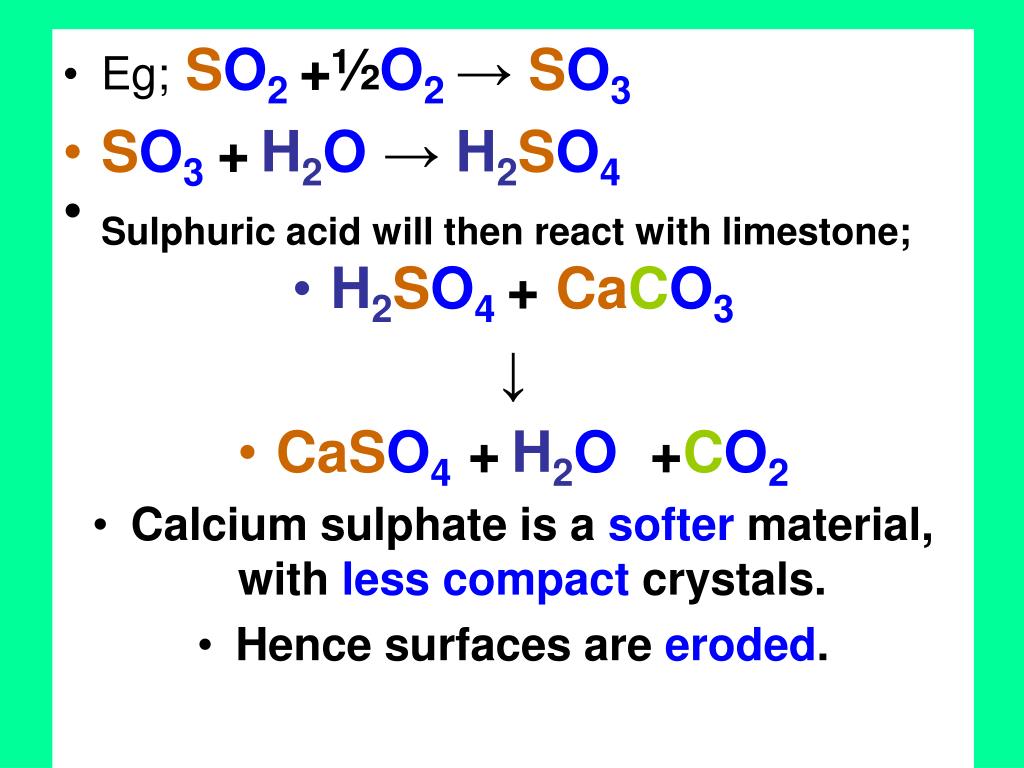
SOLVED: One of the results of acid rain is the reaction of sulfuric acid, H2SO4, with calcium carbonate, CaCO3, to produce calcium sulfate, CaSO4, and carbonic acid, H2CO3. The chemical equation for

![Solved Question 1 [15] During a production process, a waste | Chegg.com Solved Question 1 [15] During a production process, a waste | Chegg.com](https://media.cheggcdn.com/study/bf1/bf1b7069-44ef-41be-856e-c8a738df0f9c/image)