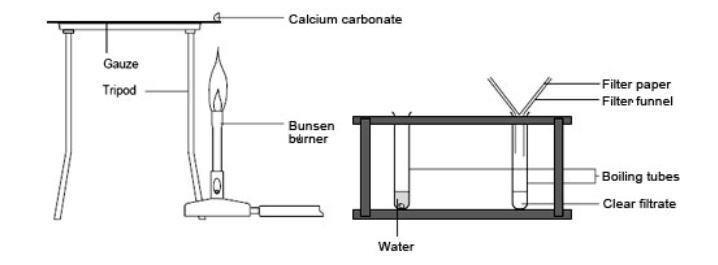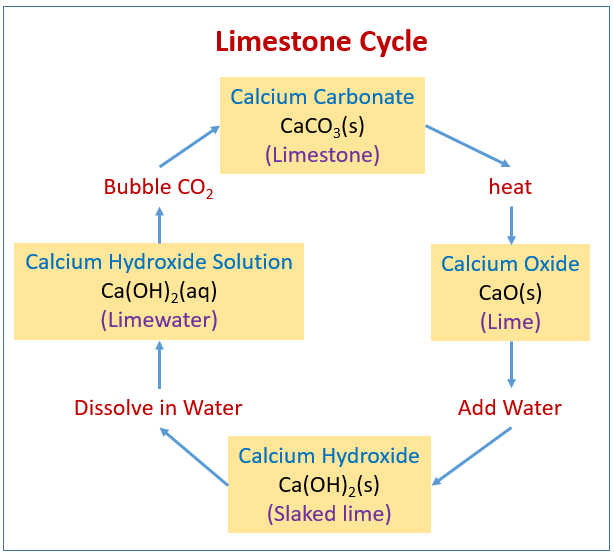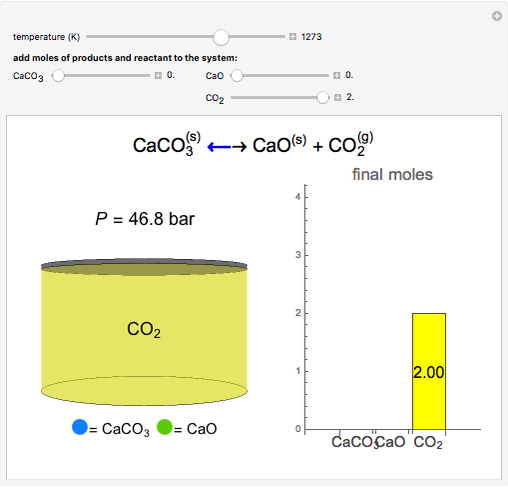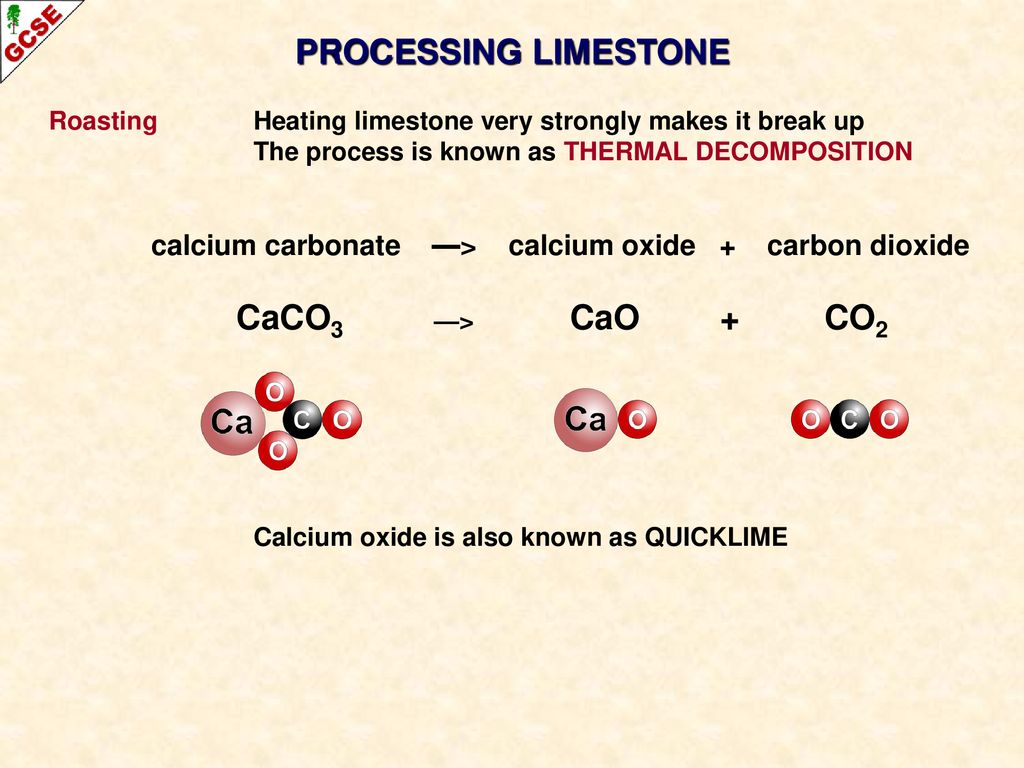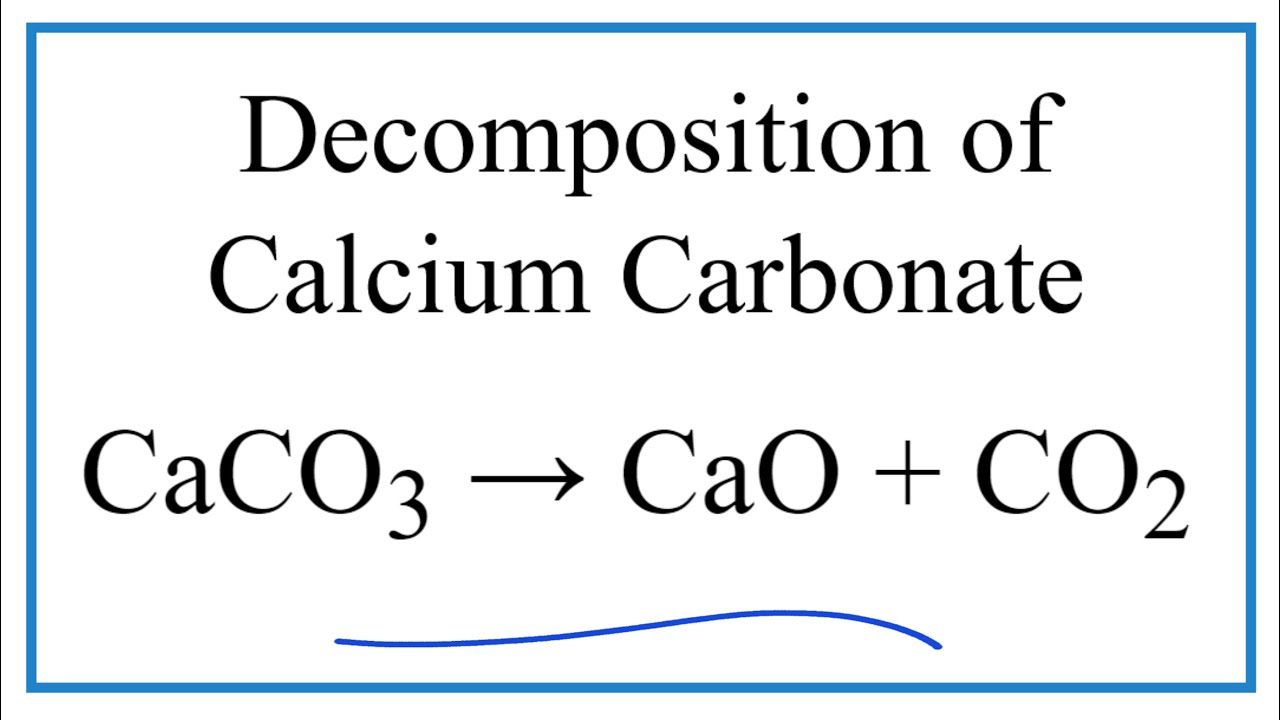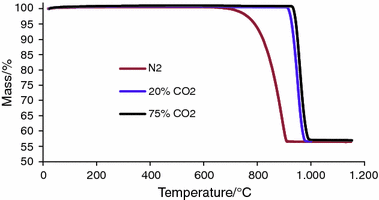
Applied Sciences | Free Full-Text | Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega

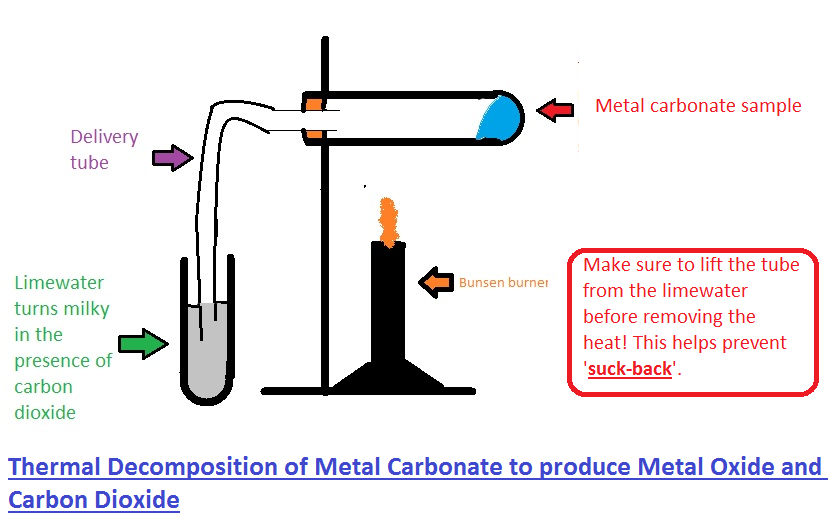
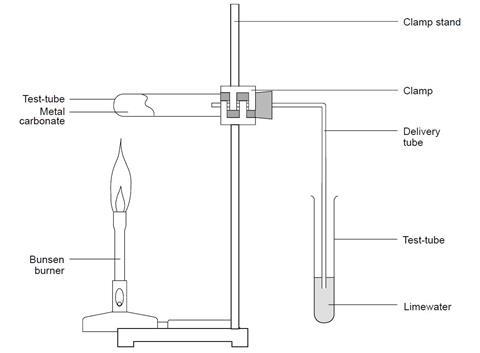
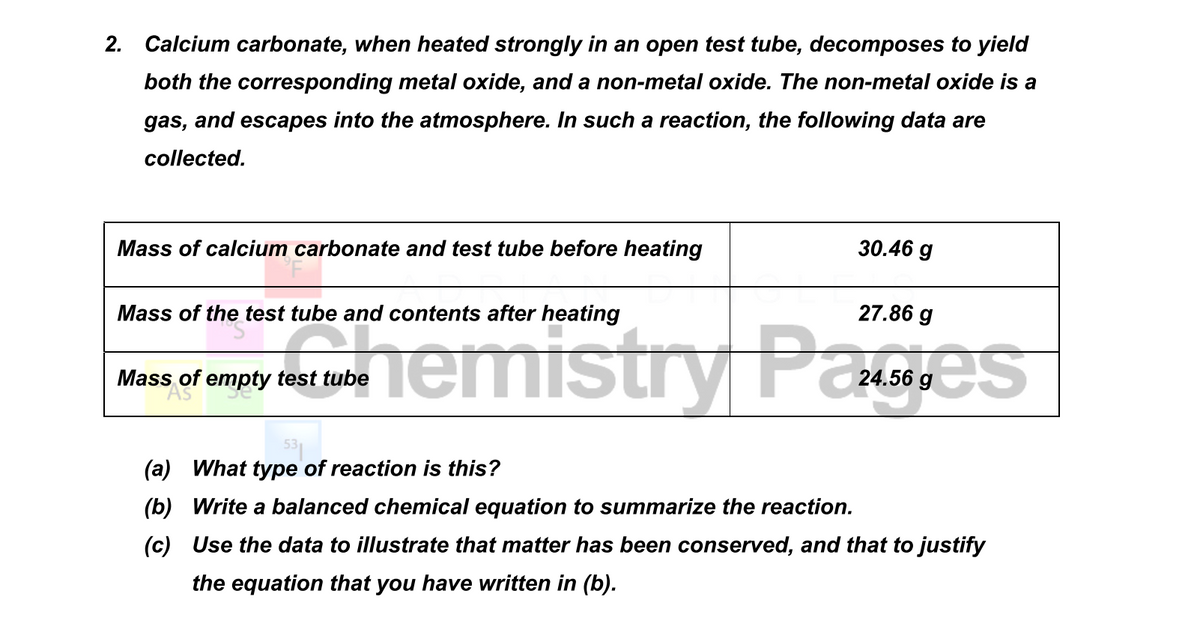
Study the Following Figure and Answer Questions.A) After Heating Calcium Carbonate, Which Gas is Formed in a Test Tube?B) When We Pass this Gas Through Limewater What Change, Did You Observe? -

Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect

50 g of an impure calcium carbonate sample decomposes on heating to give carbon dioxide and 22.4 g calcium oxide. The percentage purity of calcium carbonate in the sample is: