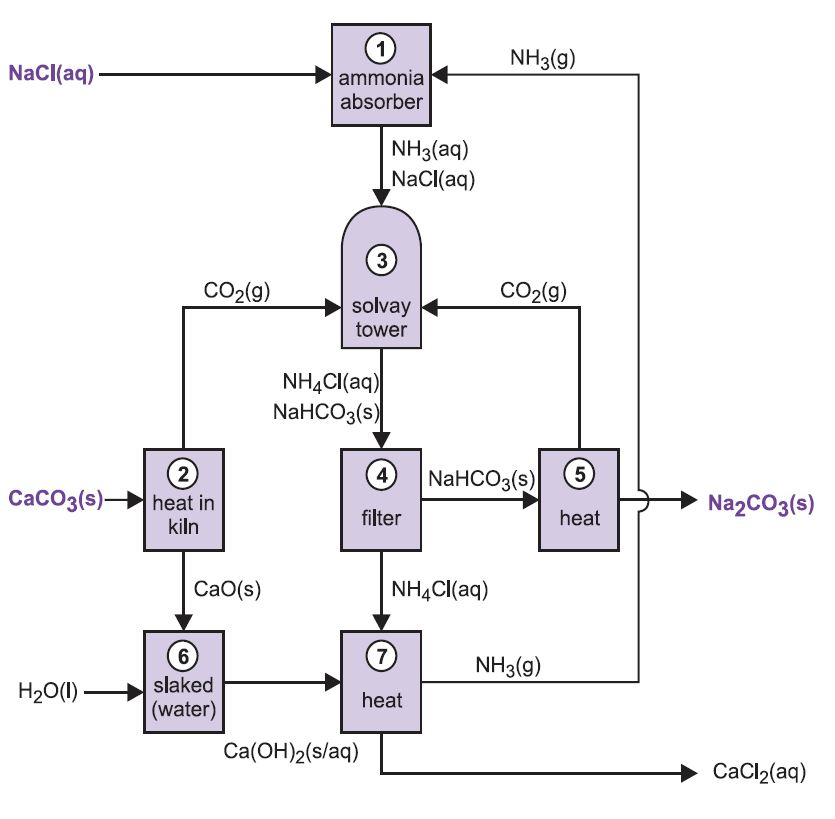
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/3-Figure1-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio
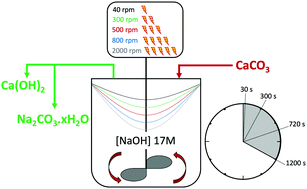
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing)
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/4-Figure2-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar
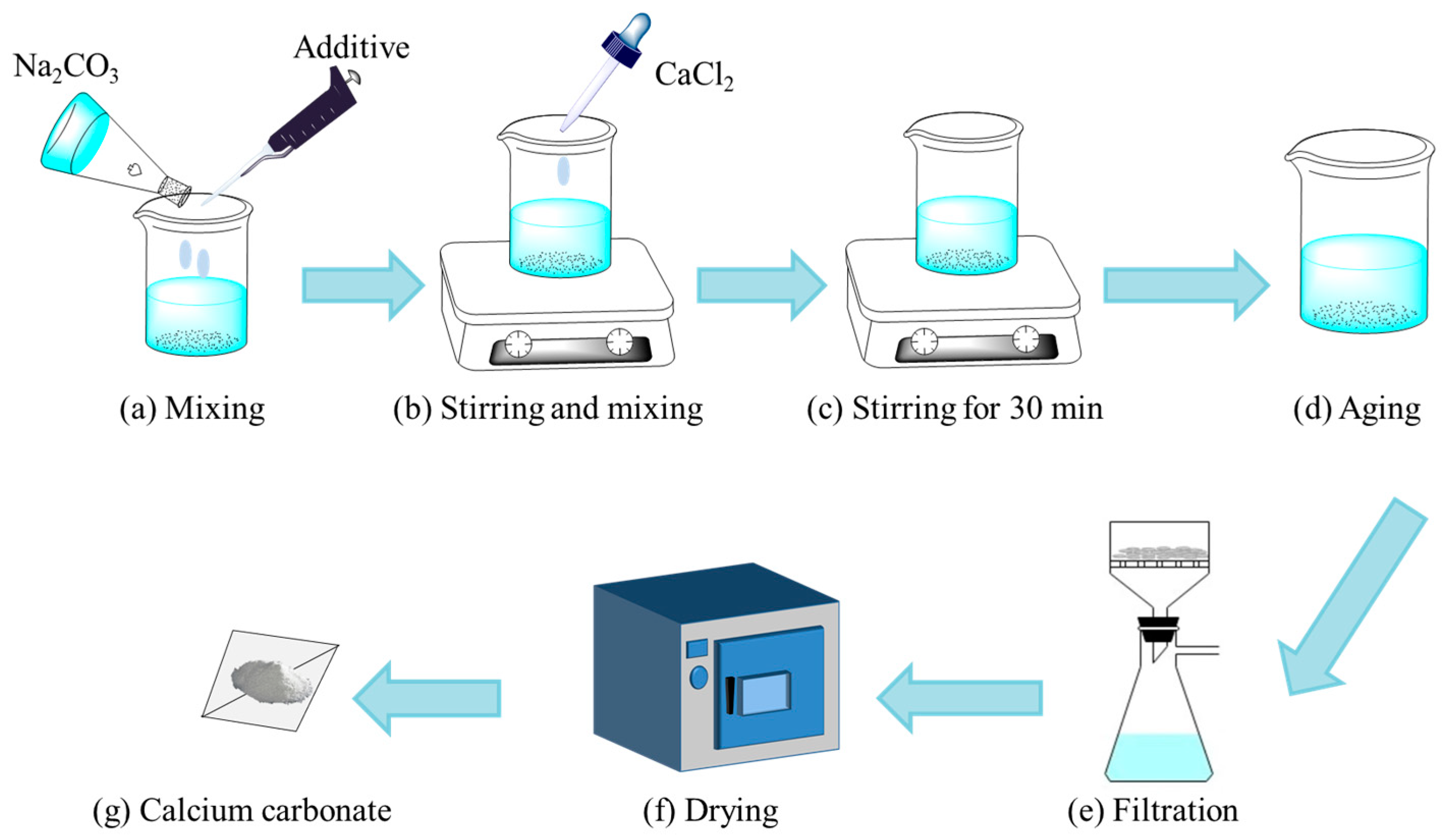
Crystals | Free Full-Text | Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing

Figure 3 from Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar

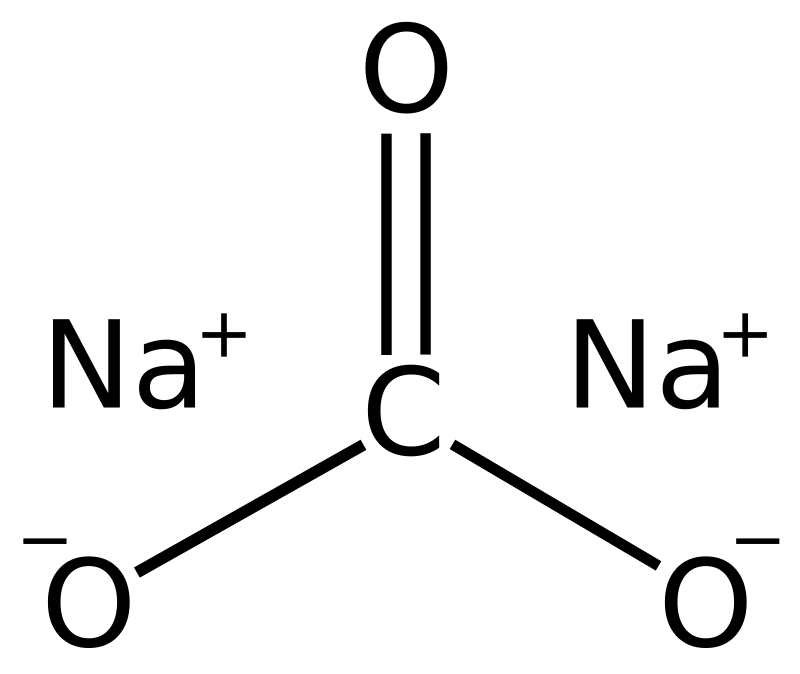
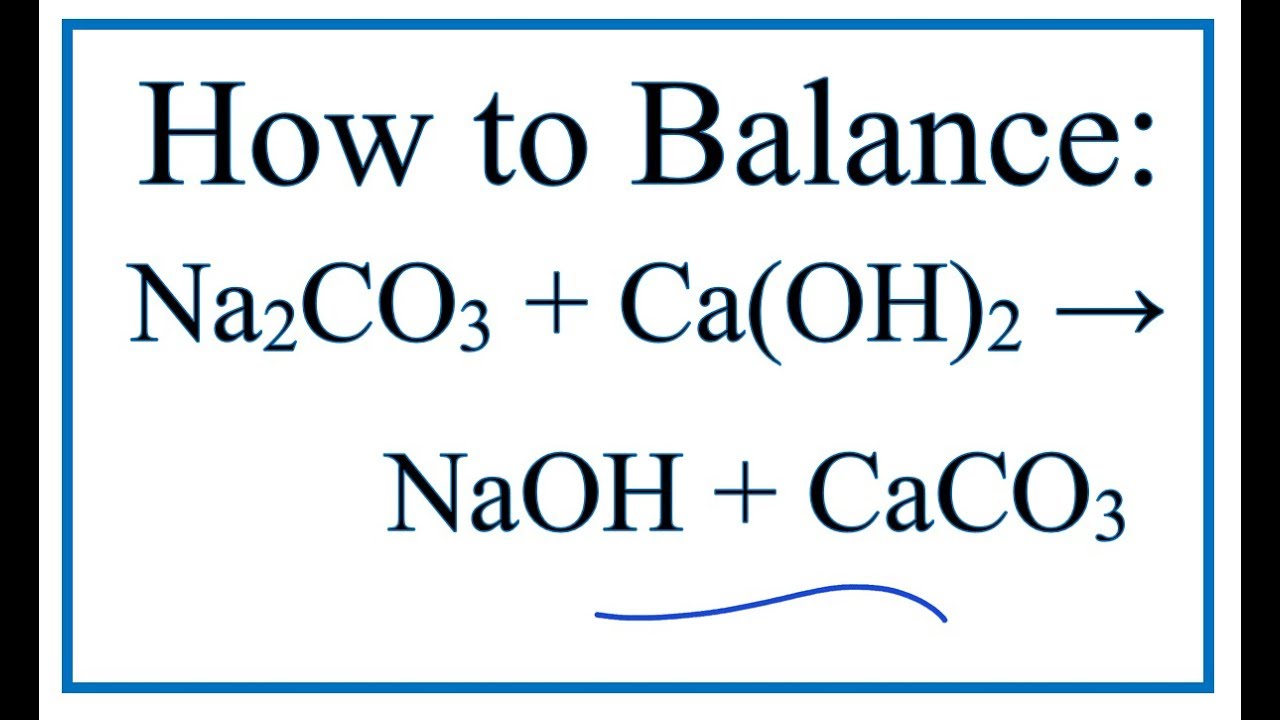
SOLVED: If 3.5 moles of sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2CO3 + Ca(OH)2 NaOH + CaCO3
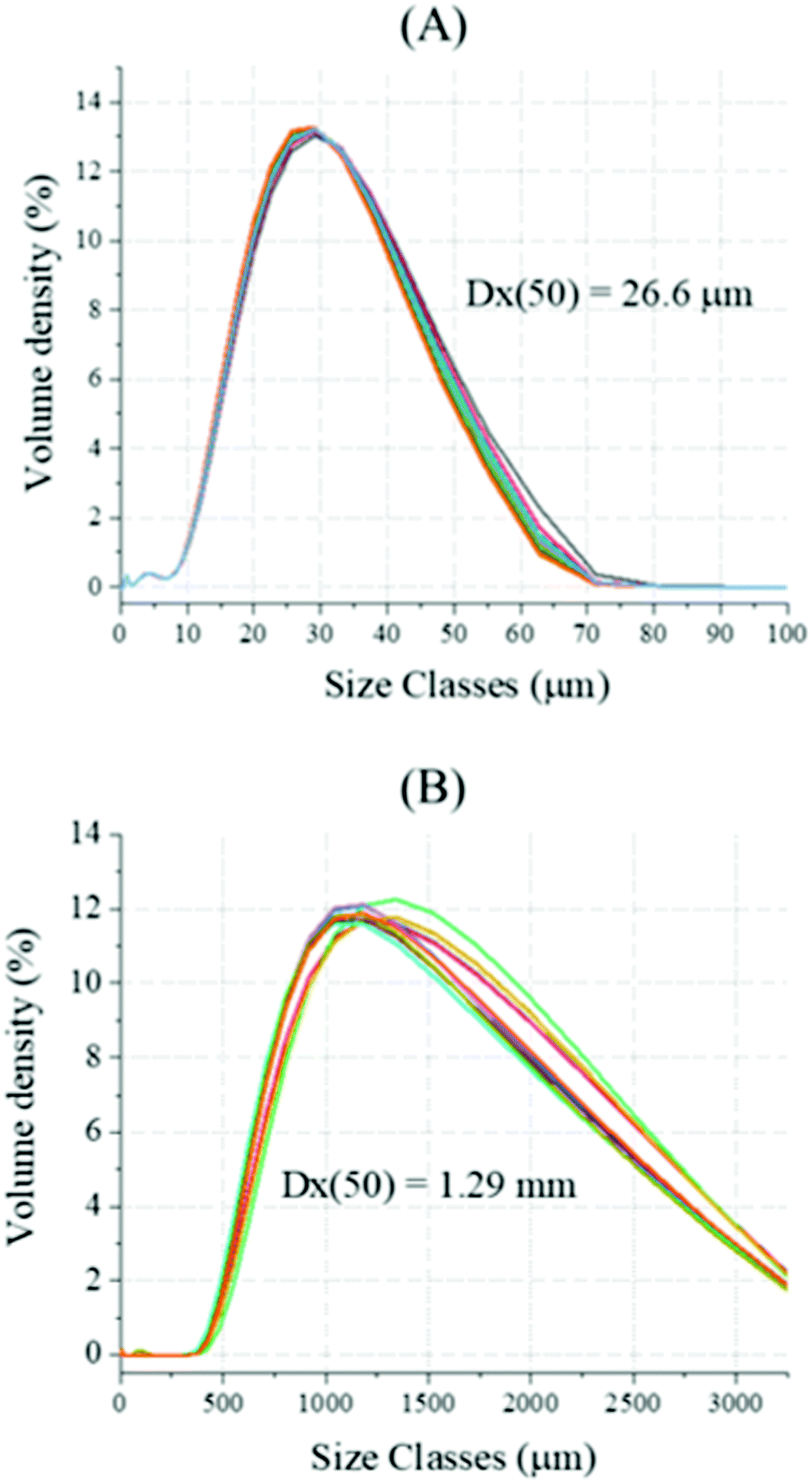
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP01412B

Look figure 4.1 and answer the following question.(a) What change would you observe in the calcium hydroxide solution taken in tube B? (b) Write the reaction involved in test tubes A and
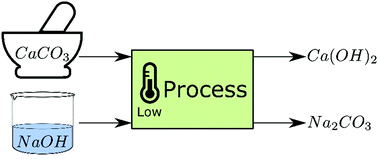
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)

Bases. Jars containing calcium carbonate (Ca2CO3), copper oxide (CuO) and sodium hydroxide (NaOH). These compounds are classified as bases, because th Stock Photo - Alamy
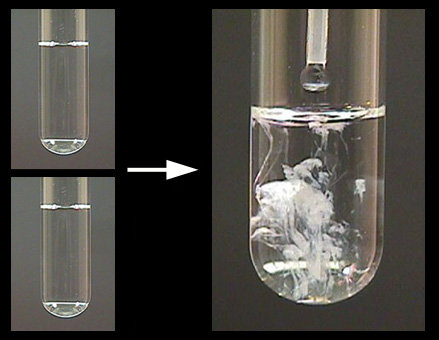
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/4-Table2-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar